
Polar Vs Nonpolar Ciencias exatas, Ciência química, Físicoquímica
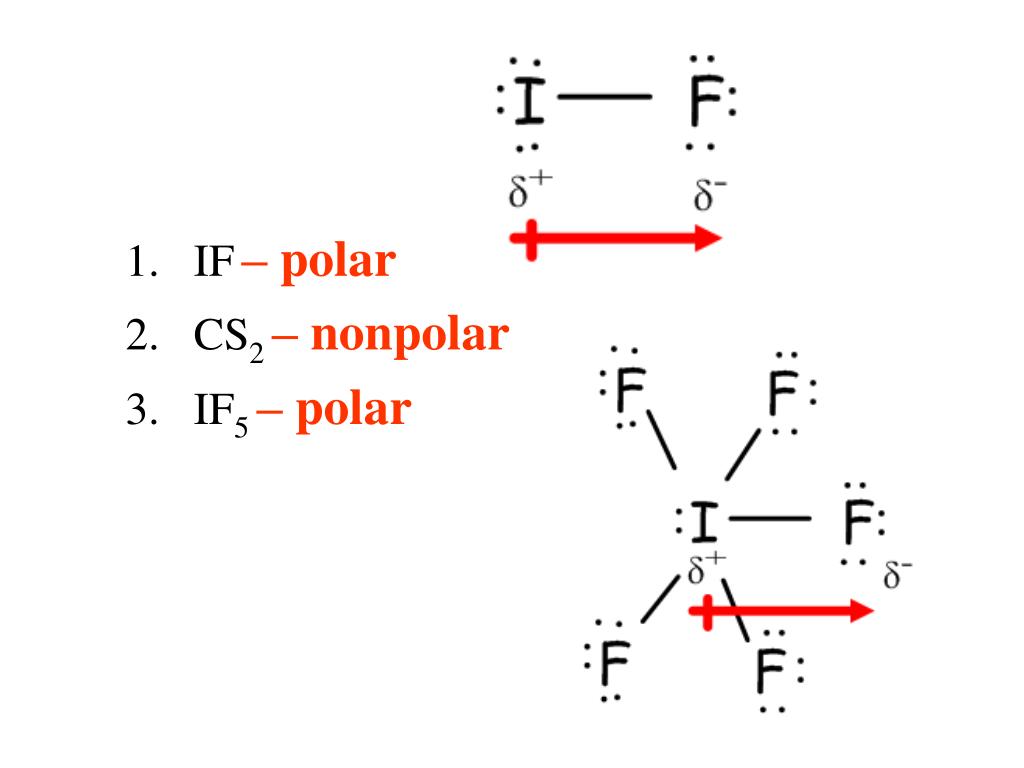
Is IF5 polar or non polar? Updated: 8/11/2023 Wiki User ∙ 9y ago Study now See answers (2) Best Answer Copy IF5 is considered a type of polar molecule. It is a polar molecule because it.

Is Hexane Polar or Nonpolar Nehemiahrilrexton
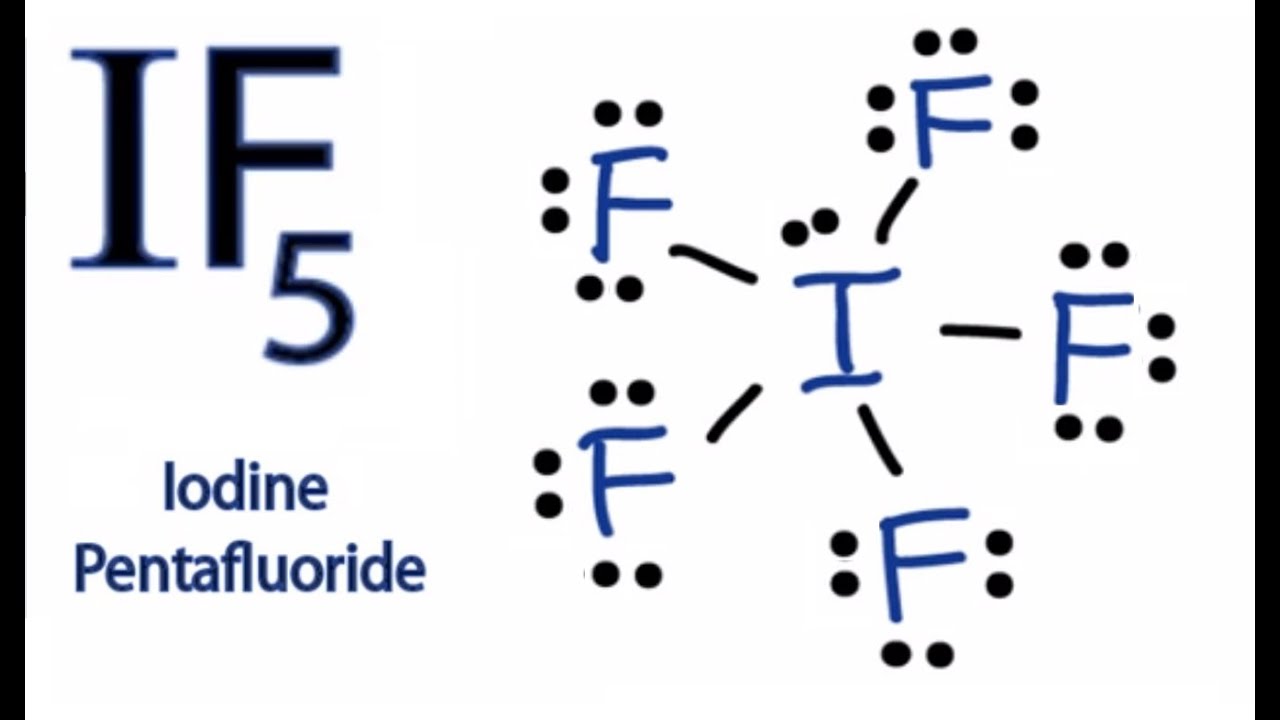
(Explained in 3 Steps) IF5 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of IF5 lewis structure and it contains five I-F bonds.

Is HF Polar or Nonpolar? (Hydrofluoric Acid) YouTube
a. SiCl4 b.. | Channels for Pearson+ Next General Chemistry 13. Liquids, Solids & Intermolecular Forces Molecular Polarity 7:17 minutes Problem 52 Textbook Question Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 Verified Solution 7m

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 สรุป
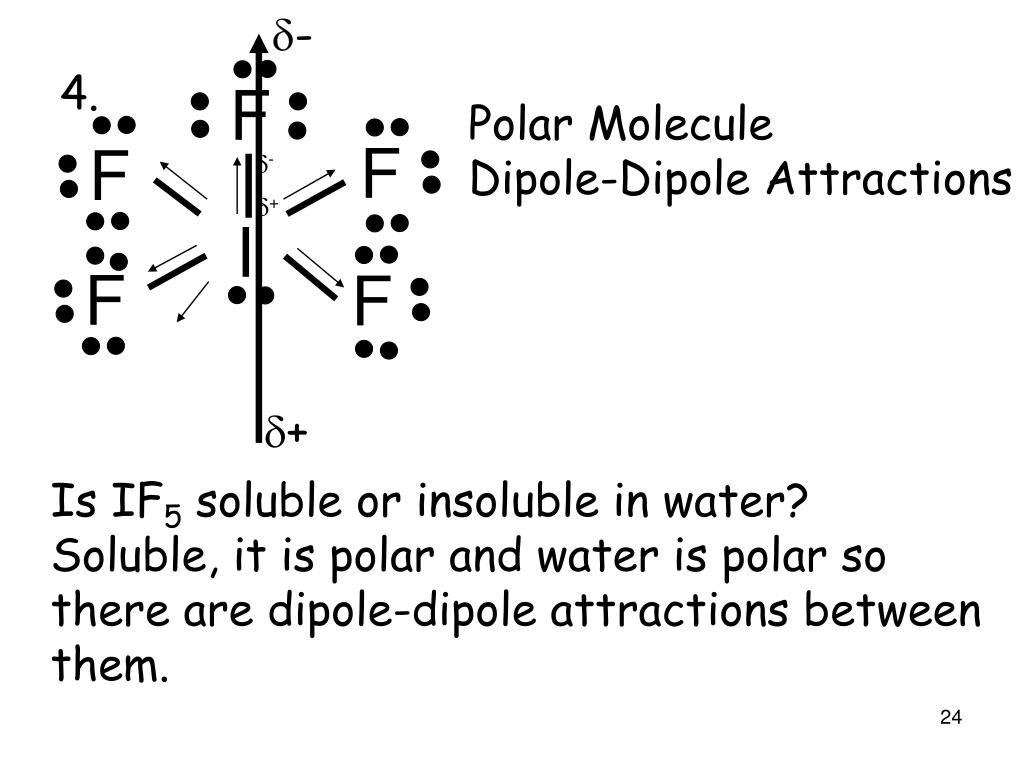
So, Is IF5 Polar or Nonpolar? IF5 is polar in nature. The molecule has a bent shaped geometrical structure because of lone pair and bond pair repulsion as per VSEPR theory due to which there occurs an imbalance in charge distribution across the molecule.
MakeTheBrainHappy Is IF5 Polar or Nonpolar?
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
What Is The Difference Between Lewis Structure And Vsepr
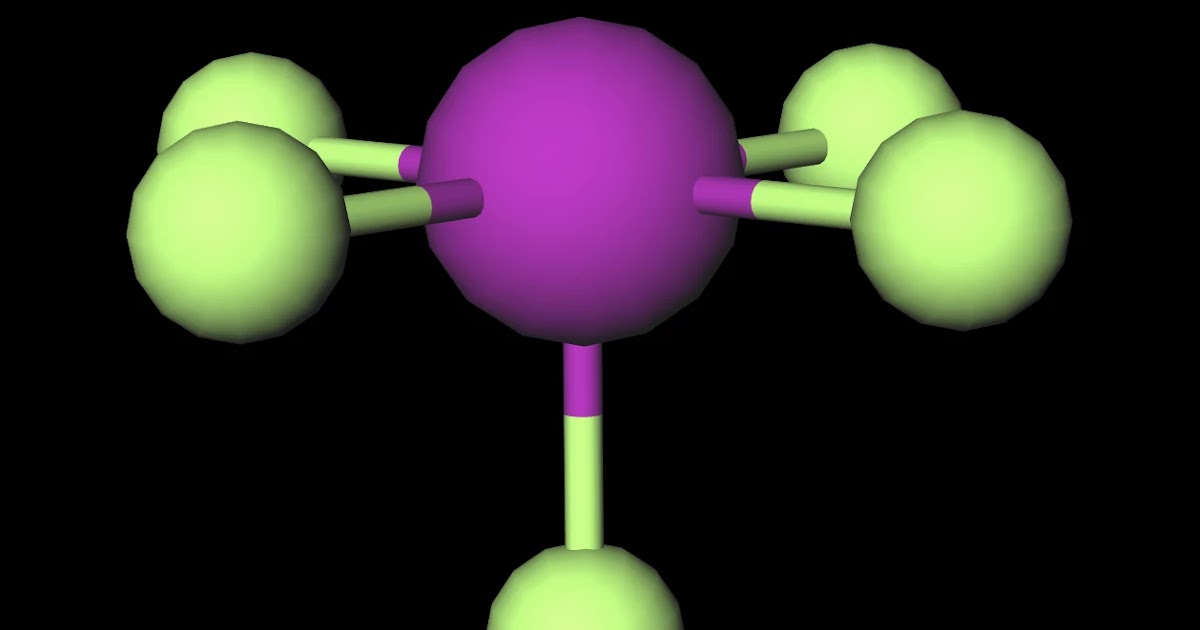
Iodine pentafluoride (IF5) is a polar molecule. The central iodine (I) atom in IF5 is surrounded by five fluorine (F) atoms forming a square pyramidal shape. The electronegativity of the fluorine (F) atom is greater than the iodine (I) atom.
PPT Part 09 IMFA and Solubility PowerPoint Presentation, free
Is IF5 Polar or Nonpolar? Answer: IF5 is a polar molecule due the presence of a lone pair of electrons which due to electron-electron repulsion results in a bent structure. This leads to an unequal distribution of charge within the molecule and therefore a permanent dipole.

Is IF5 Polar or Nonpolar? Techiescientist
100% (11 ratings) Part k The molecule. View the full answer Transcribed image text: Part A Draw an appropriate Lewis structure for IFS. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone Q (5 ଡ BO Identify the geometry of IF5 using VSEPR theory.
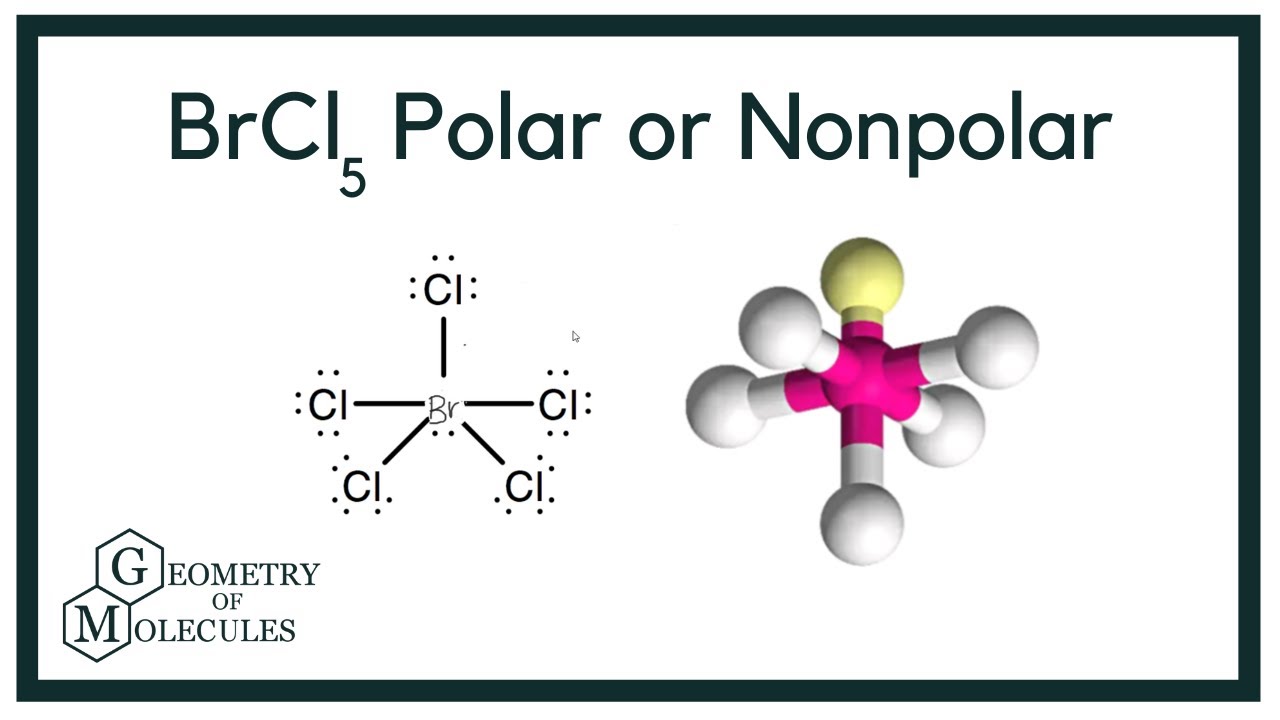
Is BrCl5 Polar or Nonpolar? (Bromine Pentachloride) YouTube
Chemistry Chemistry questions and answers Specify whether the molecule IFs is polar or nonpolar and explain why. The molecule is polar because all the I-F bonds are polar and the net dipole moment is nonzero. The molecule is polar only because all the I-F bonds are polar. The molecule is nonpolar because all the I-F bonds are nonpolar.
PPT Unit 79 Test Review PowerPoint Presentation, free download ID
Page Contents show How to draw lewis structure of IF5? The Lewis structure of iodine pentafluoride (IF5) consists of iodine (I) atom at the center. It is bonded to five atoms of fluorine (F) at the sides. There are a total of 6 electron pairs around the central iodine atom in the IF5 lewis dot structure.
Cf Polar Or Nonpolar Slidesharetrick My XXX Hot Girl
BrF3, or bromine trifluoride, is a powerful fluorinating agent for chemical reactions with sp3d hybridization in its center bromine atom. It's a T-shaped molecule with an 86.2° bond angle. The molecule is very polar, and it is mostly utilized to make uranium hexafluoride during uranium processing.

Hexano é Polar Ou Apolar AskSchool
There are four electron groups around the central atom. As shown in Figure 9.2.2 9.2. 2, repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 109.5°. 3. All electron groups are bonding pairs, so the structure is designated as AX 4.
Grade 11 CHAPTER 3 BONDING IN SIMPLE MOLECULES SEMESTER 1
IF5 has a molecular mass of 221.9 grams per mole and a density of 3.25 g/cm3. It is a melt temperature of -28.8degC, and the boiling point is 47.3degC. The IF5 molecule is polar because of its asymmetrical structure. It comprises an iodine atom centrally bonded to five fluorine molecules in a bipyramidal trigonometric arrangement.

Is If5 Polar Or Nonpolar
Guide Is If5 Polar Or Nonpolar September 22, 2022 Webster West The Iodine pentafluoride chemical formula is IF5. Drawing IF5 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct IF5 Lewis Structure.
[Solved] image attached 1. Complete the table below. Indicate whether
The Lewis Structure (Lewis Dot Diagram) for IF5.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out.